Abstract
Introduction
B-cell prolymphocytic leukemia (B-PLL) is a rare mature lymphoid neoplasm. It was first described as a variant of chronic lymphocytic leukemia, but is now a distinct entity of the WHO classification. Diagnosis requires the presence of more than 55% prolymphocytes in the peripheral blood. The lack of specific phenotypical signature makes B-PLL diagnosis challenging, with an overlap with mantle cell lymphoma. Limited information is currently available on this rare disease and there is no consensus on treatment strategy. The aim of this study is to better characterize clinical features, treatment and outcome of patients with B-PLL We retrospectively included patients who were diagnosed with de novo B-PLL, with clinical data available. All blood smears at diagnosis were reviewed by three independent expert cytologists. Clinical characteristics were retrospectively abstracted from medical records. Response was evaluated using IWCLL criteria. Kaplan-Meier estimates of overall and progression-free survival rates were obtained and analyzed according to 17p deletion status (log-rank test).
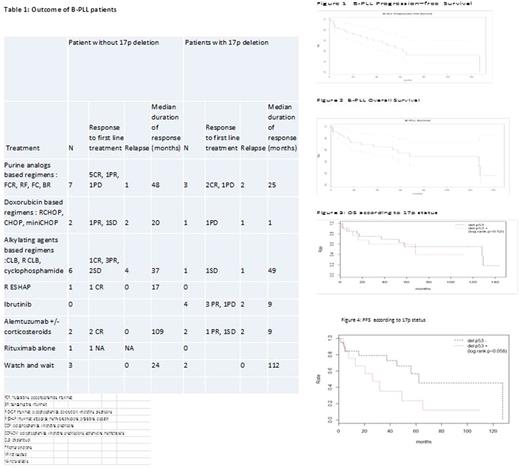
Results : Blood smears of 85 patients with a suspected B-PLL diagnosis were sent for reviewing. Only 36 (42%) strictly met the WHO diagnostic criteria according to the three cytologists. One patient was excluded due to the absence of any clinical data available. The 35 patients included in the study were diagnosed with B-PLL in 15 French Hematological Departments between 1992 and 2017. Median age at diagnosis was 73 years old (IQR, 65-79), with a predominance of male (57%, n=20). Fifteen patients (42%) presented at least one comorbidity. At diagnosis lymph nodes were present in most half of the patients (49%, n=17), splenomegaly in 10 patients (29%) and B symptoms in only 3 patients (9%). Richter syndrome was present in 2 patients at diagnosis of B-PLL, one with a diffuse large B cell lymphoma and the second one with a Burkitt lymphoma. As far as the biological presentation is concerned, median lymphocytosis at diagnosis was 25 G/L (IQR, 14-53) with a median of 80% (IQR, 71-90) of prolymphocytes. Median hemoglobin level and platelet count were 12g/dL (IQR, 10-14) and 141 G/L (IQR, 100-187) respectively. LDH were elevated in 12 patients (67%) and normal in 6, with a median value of 510 UI/L (IQR, 399-969). Median value of beta-2-microglobulin was 3 mg/L (IQR, 2-6). Regarding cytogenetics, 14 patients (40%) had a 17p deletion either by karyotype or by FISH, and the majority (n=25, 71%) had a complex karyotype (≥3 abnormalities)
Thirty patients (86%) received treatment in a median delay of 3 months after diagnosis and a watch and wait strategy was preferred in 5 patients. First-line treatment consisted of systemic chemotherapy in 22 (73%) cases and targeted drugs in 8 patients (27%), especially ibrutinib in 4 patients with 17p deletion. Fludarabine-based regimens were used in 6 patients (20%). Rituximab was part of the first-line treatment in 13 patients (43%) (table 1). First-line treatment regimens and response to treatment are detailed in Table 1. After first-line treatment, the overall response rate of patients with B-PLL was 70% [complete response, n=11 (37%); partial response (PR), n=10 (33%)]. Stable disease or progression was observed in 7 patients (23%), and response to treatment was not available in 2 patients. A partial response was observed in 3 patients out of 4 treated with ibrutinib .Two patients progressed with a median delay of 16.5 months. Relapse or progression were observed in 17 patients (49%) with a median time to progression of 16 months (IQR, 5-25).
With a median follow-up of 48 months, the overall and progression-free survival rates at 5 years were respectively of 55% (95%CI [39-78]) and 45% (95%CI [29-70]) (Figures 1, 2). There was no difference in overall survival according to 17p deletion status (p=0.52), but the data suggested a large difference in progression-free survival (Hazard Ratio HR=2.47, 95%CI [0.94-6.49], p=0.058) ( Figures 3,4).
Conclusion : We report the largest retrospective study of patients with B-PLL treated with heterogeneous treatments in France from 1992 to 2017. The median of median OS was 70 months. Seventeen p deletion is associated with a shorter progression-free survival duration. The impact of novel agents used in high -risk CLL on response duration and overall survival should be assessed in an international prospective trial regarding the rareness of the disease.
Feugier: Roche: Consultancy, Honoraria, Research Funding. Fornecker: Takeda: Honoraria; Roche: Honoraria; Gilead: Honoraria; Servier: Honoraria; BMS: Honoraria. Leblond: Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.